Write the Full Orbital Diagram for F
Part A Write the full orbital diagram for F. 0 Correct Part G Write full orbital diagram for.

Write The Full Orbital Diagram For Fluorine Study Com
B Indicate the number of unpaired electrons in it.
. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Orbital diagram of Sodium Na 12. The first number is the principal quantum number n and the letter represents the value of l angular momentum quantum number.
Orbital diagram of Nitrogen N 8. Express your answer as an integer. Start studying Orbital Diagrams.
Click within the orbital to add electrons. The orbital for a hydrogen atom. Write the full orbital diagram for F.
Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. Orbital diagram of Carbon C 7. Express your answer as an integer.
Students whove seen this question also like. Ar 4s 2 3d 1 2. Write orbital diagrams for the valence electrons and indicate the number of.
Labels can be used once more than once or not at all. Drag the appropriate labels to their respective targets. SOLVEDWrite the full orbital diagram for each element.
N Write electron configuration and use the symbol of the previous noble gas in. Write the full orbital diagram for each element. Use the buttons at the top of the tool to add orbitals.
By signing up youll get thousands of. Therefore the lithiumLi electron configuration will be 1s 2 2s 1. Orbital diagram of Oxygen O 9.
Problem 52 Medium Difficulty. So the next six electrons enter the 2p orbital and the remaining one electron enter the 3s orbital. Drag the appropriate labels to their respective targets.
Drag the appropriate labels to their respective targets. Write the full orbital diagram for element. Learn vocabulary terms and more with flashcards games and other study tools.
Scandium 1s 2s 2p 3s 3p 4s 3d Full electron configuration. How to write the orbital diagram for lithiumLi. Drag the appropriate labels to their respective targets.
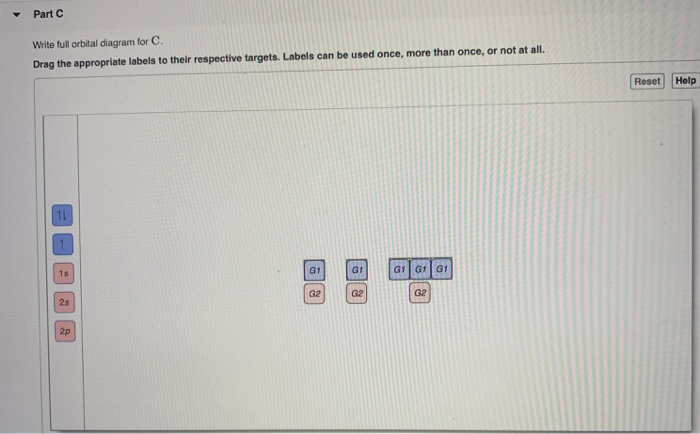
Reset Help 11 1s G1 G1 G1 G1 G1 G2 G2 G2 2s 2p Write the full orbital diagram for C. Labels can be used once more than once or not at all. Part C Write the full orbital diagram for B.
It has one arrow in it facing up. Write full orbital diagrams and indicate the number of unpaired electrons for. Labels can be used once more than once or not at all.
Labels can be used once m. To create an orbital diagram of an atom you first need to know Hunds principle and Paulis exclusion principle. Click within the orbital to add electrons.
A Write the full orbital diagram for F. Draw orbital diagrams for the following elements. TL 1s 11 11 11 1 1 1s 2s 25 2p 2p Part B Indicate the number of unpaired electrons in F.
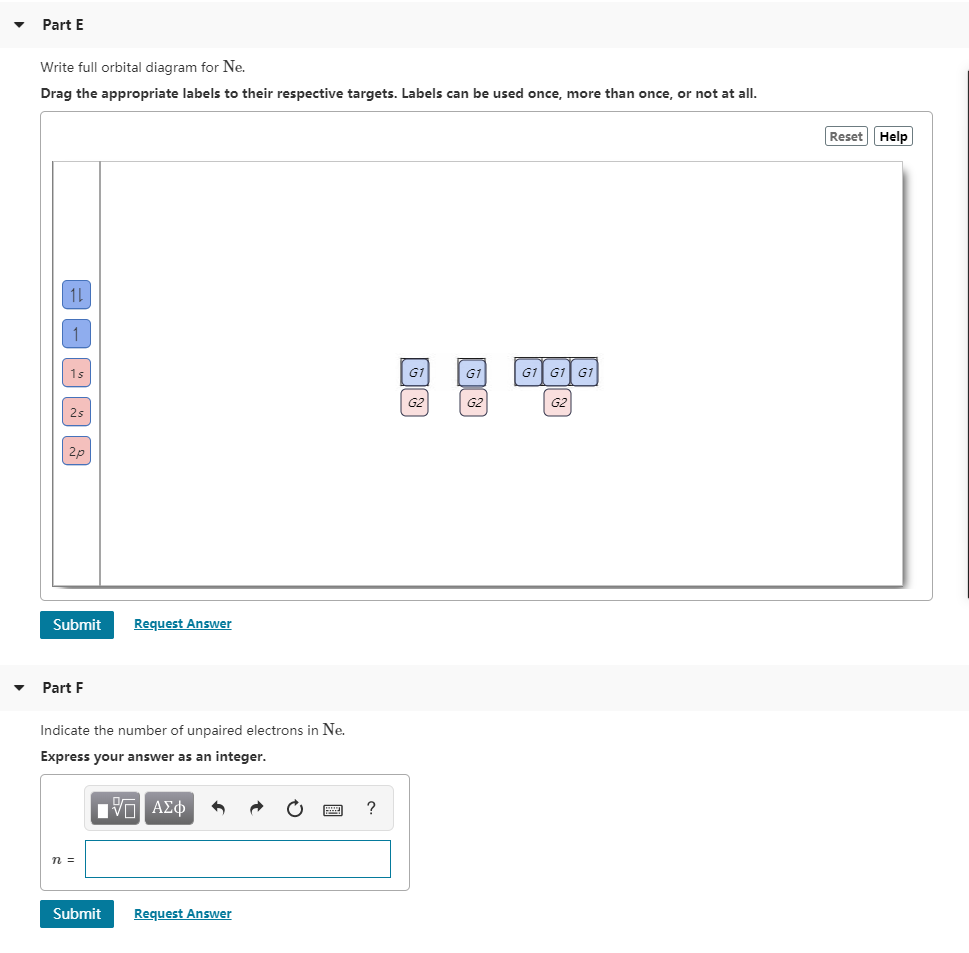
Orbital diagram of Neon Ne 11. It symbolizes the electron as an arrow in a box that represents the orbital. Write full orbital diagrams and indicate the number of unpaired electrons fo 0200.
Is a box with 1s under it and an H next to it. Write full orbital diagrams and indicate the number of unpaired electrons for each element. N Part G Write full orbital diagram for Be Use the buttons at the top of the tool to add orbitals.
The s-orbital can have a maximum of two electrons. Labels can be used once more than once or not at all. Part C Write the full orbital diagram for B.
Part H Indicate the number of unpaired electrons in it. How to write the orbital diagram for sodium. Write the full orbital diagram for each element.
Drag the appropriate labels to their respective targets. Not all targets will be filled. Orbital diagram of Boron B 6.
Express your answer as an integer. Correct Part F Indicate the number of unpaired electrons in it. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1 Core notation.
Orbital diagram of Lithium Li 4. A mathrmF b C c Ne d Be. Write orbital diagrams for the valence electrons and indicate the number of.
Want to see the full answer. Find step-by-step Chemistry solutions and your answer to the following textbook question. Therefore the sodiumNa electron configuration will be 1s 2 2s 2 2p 6 3s 1.
To create an orbital diagram of an atom you first need to know Hunds principle and Paulis exclusion principle. The remaining five electrons will go in the 2p orbital. Write the electron configuration full and in core notation.
Therefore the remaining one electron enters the 2s orbital. Orbital diagram of Fluorine F 10. Write the full orbital diagram for element.
Want to see the full answer. AntimonySb is the 51st element in the periodic table and its symbol is Sb. An orbital diagram is a different way to show the electron configuration of an atom.
Check out a sample QA here. 1 s 2 p 3 d and 4 f for the orbital and the superscript number tells you how many electrons are in that orbital. Therefore the F electron configuration will be 1s 2 2s 2 2p 5.
Orbital diagram of Beryllium Be 5. Video Player is loading. This article gives an idea about the electron configuration of antimony and orbital diagram period and groups valency and valence electrons of antimony bond formation compound formation.
Orbital diagrams use the same basic format but instead of numbers for the electrons they use and arrows as well. Orbital diagram of Magnesium Mg 13. Check out a sample QA here.
Solved Exercise 9 52 Part A Write Full Orbital Diagram For Chegg Com

Solved Part A Write Full Orbital Diagram For F Drag The Chegg Com
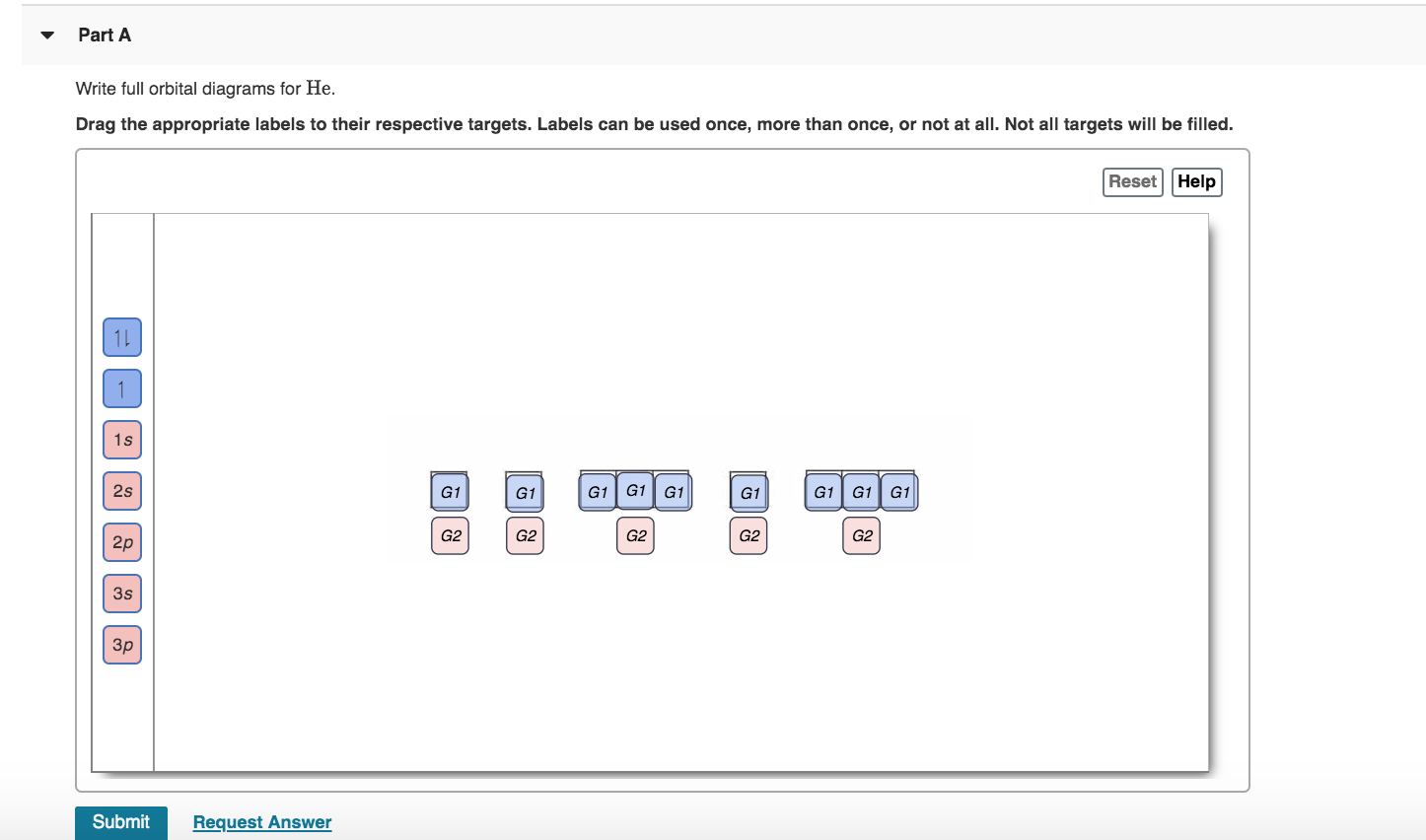
Solved Part A Write Full Orbital Diagrams For He Drag The Chegg Com
Comments
Post a Comment